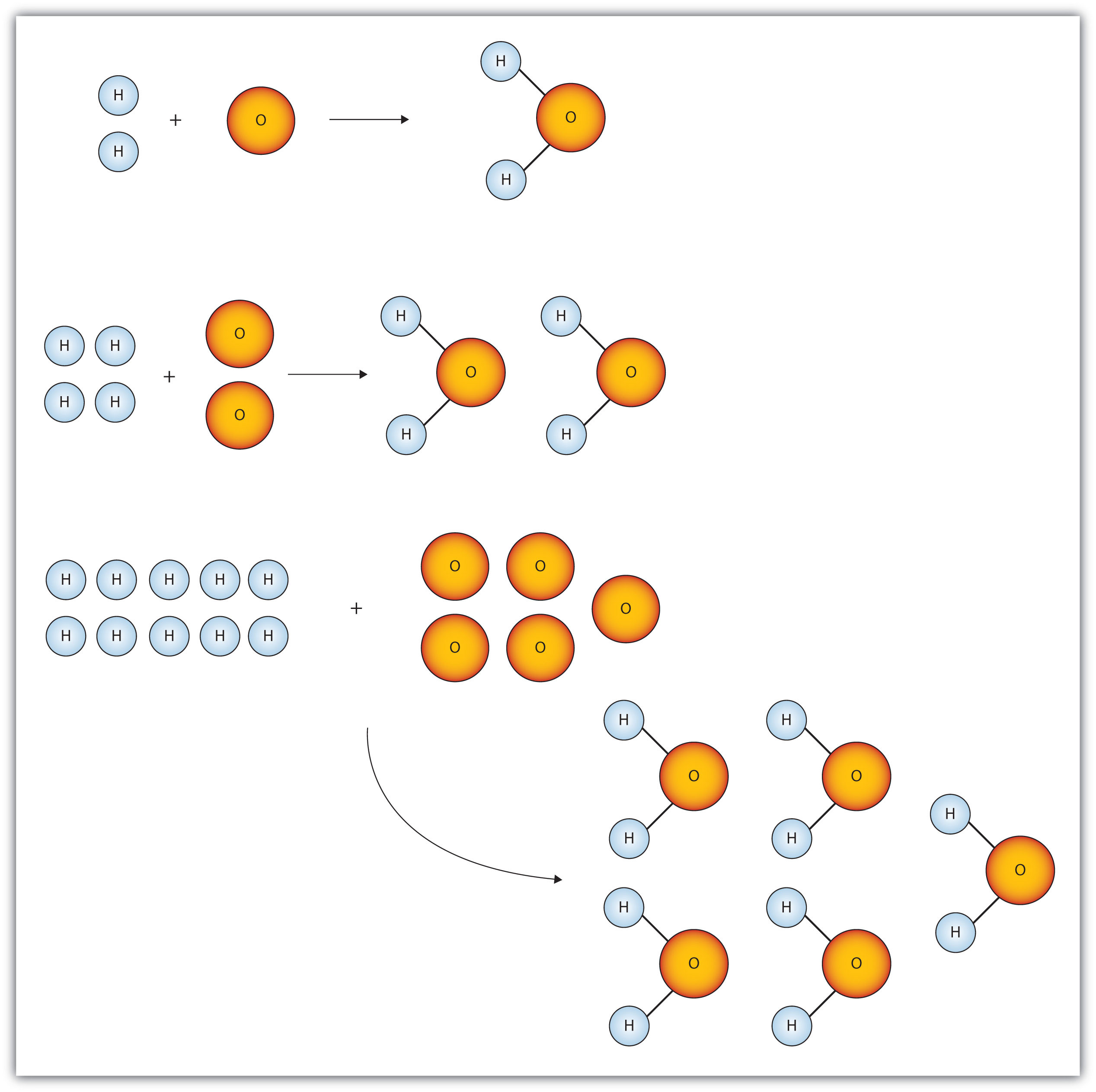
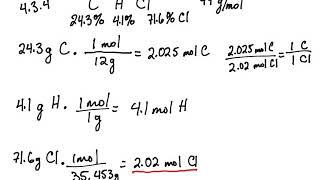Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download
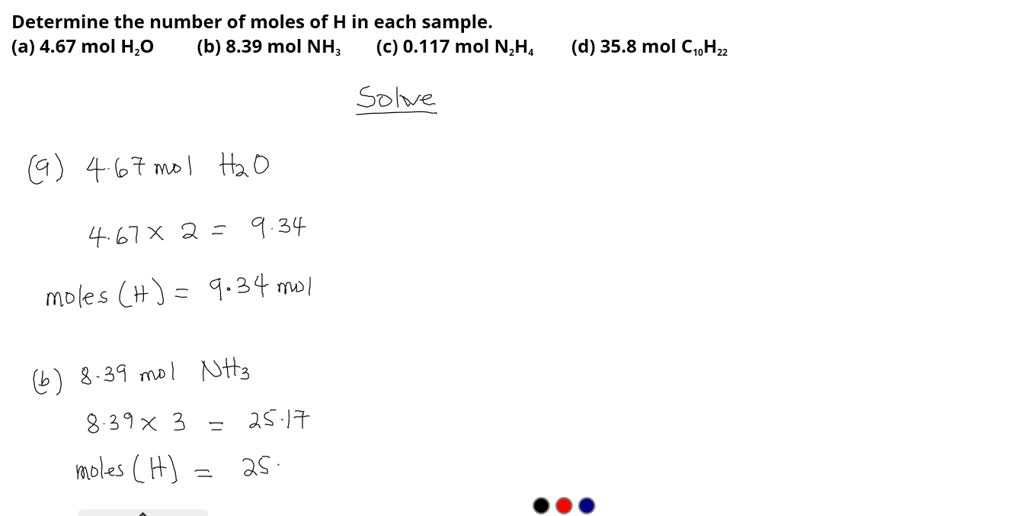
SOLVED: Determine the number of moles of H in each sample.(a) 4.67 mol H2O (b) 8.39 mol NH3(c) 0.117 mol N2H4 (d) 35.8 mol C10H22

Chemistry Warm Up: Mole / Mass / Particles 1.What is the mass of one mole of water? 2.If one milliliter of water has a mass of 1.00grams, how many moles. - ppt download
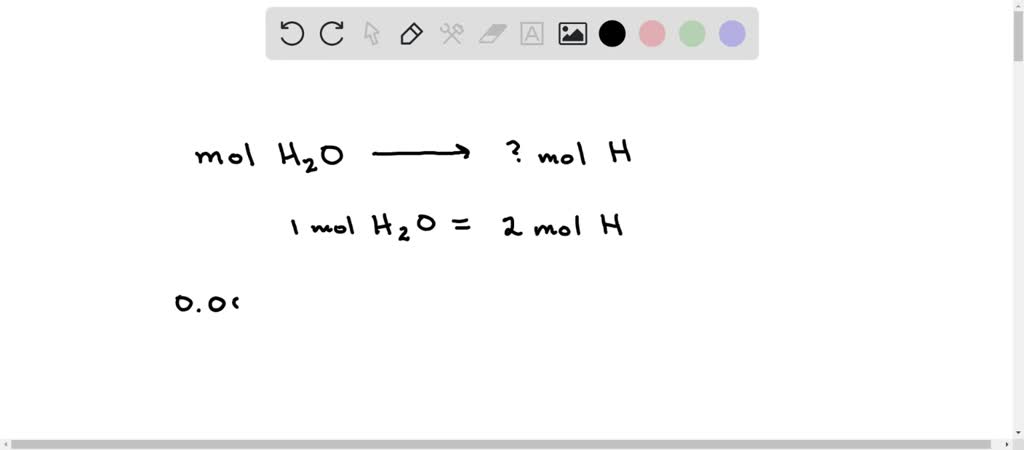
SOLVED: During the analysis, 0.00905 mol H2O is formed. Calculate the amount (mol) H in 0.00905 mol H2O.
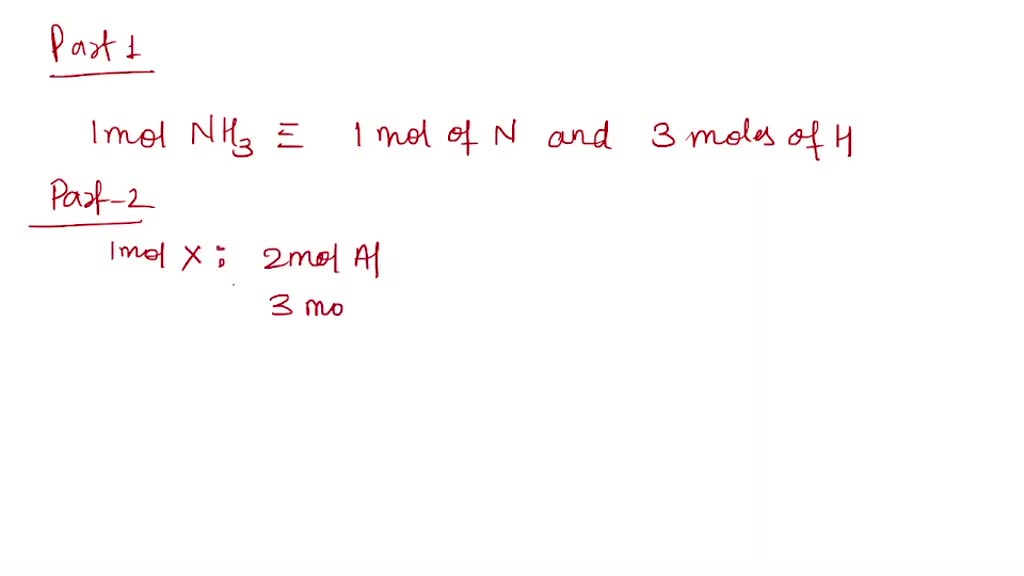
SOLVED: Part 1 (2 points) #d See Periodic Table See Hint 1mol NH3 contains mol Nand molH Part 2 (1 point) Write the chemical formula for 1 mol of compound X containing
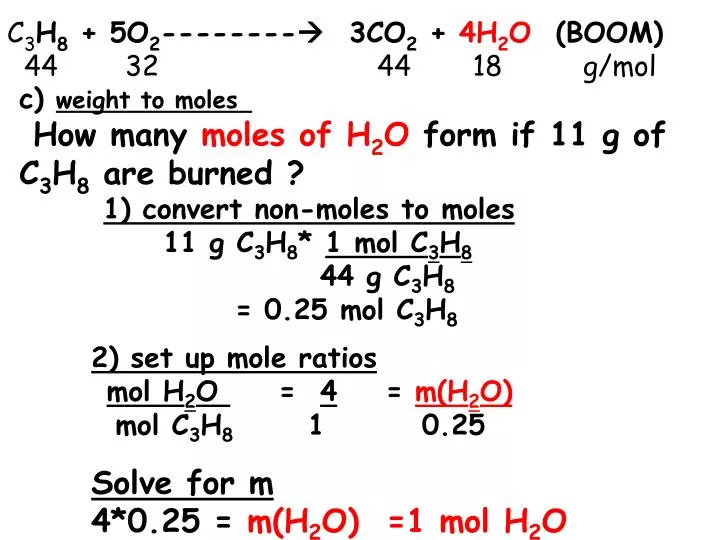
PPT - c) weight to moles How many moles of H 2 O form if 11 g of C 3 H 8 are burned ? PowerPoint Presentation - ID:2012424

1 H NMR spectrum of imidazole in deuterated chloroform solution (0.1... | Download Scientific Diagram
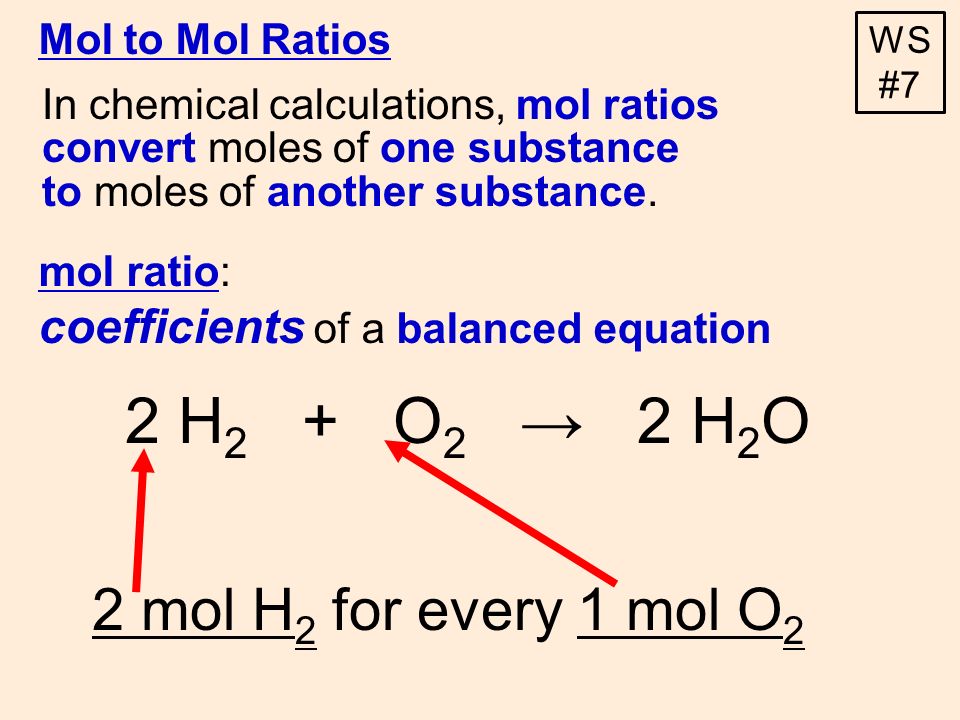
Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download

The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download





![Solved Given that delta H^degree_f [H(g)] = 218.0 kJ.mol^-1 | Chegg.com Solved Given that delta H^degree_f [H(g)] = 218.0 kJ.mol^-1 | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F1a0%2F1a02aac8-2249-4506-869f-c847df3f3774%2FphpydBdke.png)